Phenolphthalein reaction with base
Phenolphthalein Reaction With Base. It is a weak acid which can lose h ions. As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. Chelonae which give a positive reaction from other rapid growers. Furthermore one of the ph testers is phenolphthalein which is usually colourless but on coming in contact with aid or base changes its colour from pink acidic to purple base.
 Phenolphthalein 77 09 8 From chemicalbook.com
Phenolphthalein 77 09 8 From chemicalbook.com
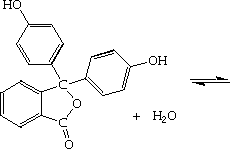
The 3 day test is used to identify and distinguish some rapid growers m. Adding hydroxide ions oh as found in bases will change the phenolphthalein into its ion and turn the solution pink. It is a weak acid which can lose h ions. Phenolphthalein is often used as an indicator in acid base titrations. As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments.
Phenolphthalein is a weak acid and is colorless in solution although its ion is pink.
Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles. If hydrogen ions h as found in an acid were added to the pink solution the equilibrium would switch and the solution would be colorless. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles. It is a weak acid which can lose h ions. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8. It is an organic compound that we use as an acid or base indicator.
 Source: researchgate.net
Source: researchgate.net
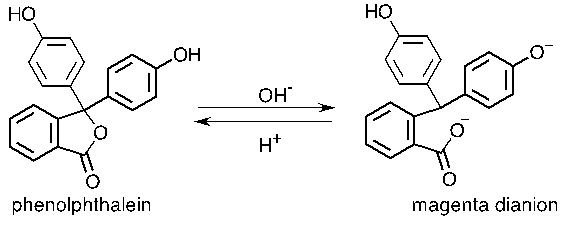
Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. Adding hydroxide ions oh as found in bases will change the phenolphthalein into its ion and turn the solution pink. The addition of a base sodium carbonate reacts with the phenolphthalein and produces a red diazo reaction that is easily visible. It is an organic compound that we use as an acid or base indicator.
 Source: researchgate.net
Source: researchgate.net
The addition of a base sodium carbonate reacts with the phenolphthalein and produces a red diazo reaction that is easily visible. Furthermore the compound is pinkish in basic solution and colourless in acidic solution. Chelonae which give a positive reaction from other rapid growers. Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. Adding hydroxide ions oh as found in bases will change the phenolphthalein into its ion and turn the solution pink.
 Source: ch.ic.ac.uk
Source: ch.ic.ac.uk
It is a weak acid which can lose h ions. Furthermore the compound is pinkish in basic solution and colourless in acidic solution. But the function of the ph indicator is far advanced. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles.
 Source: chegg.com
Source: chegg.com
Furthermore the compound is pinkish in basic solution and colourless in acidic solution. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8. Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. Phenolphthalein is often used as an indicator in acid base titrations. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles.

Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. It is an organic compound that we use as an acid or base indicator. Chelonae which give a positive reaction from other rapid growers. However phenolphthalein is no longer used as a laxative due to the suspected carcinogenicity of this compound. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles.
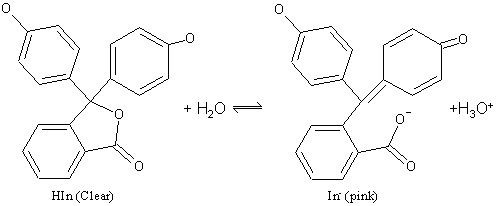 Source: digipac.ca
Source: digipac.ca
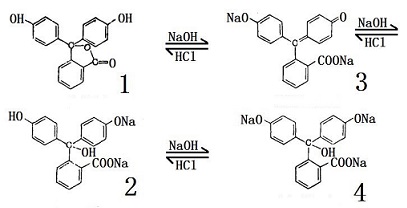
Furthermore the compound is pinkish in basic solution and colourless in acidic solution. And phenol react to give phenolphthalein which is similar in structure to fluorescein but lacks the oxygen linking two of the aryl rings. The addition of a base sodium carbonate reacts with the phenolphthalein and produces a red diazo reaction that is easily visible. Phenolphthalein is a chemical compound with the formula c20h14o4 and is often written as hin or phph in shorthand notation. It is a weak acid which can lose h ions.
 Source: quora.com
Source: quora.com
Phenolphthalein is often used as an indicator in acid base titrations. For this application it turns colorless in acidic solutions and pink in basic solutions. Phenolphthalein is a chemical compound with the formula c20h14o4 and is often written as hin or phph in shorthand notation. Furthermore one of the ph testers is phenolphthalein which is usually colourless but on coming in contact with aid or base changes its colour from pink acidic to purple base. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8.
 Source: sites.google.com
Source: sites.google.com
It belongs to the class of dyes known as phthalein dyes. If hydrogen ions h as found in an acid were added to the pink solution the equilibrium would switch and the solution would be colorless. Phenolphthalein is a weak acid and is colorless in solution although its ion is pink. As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. It is a weak acid which can lose h ions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It belongs to the class of dyes known as phthalein dyes. It belongs to the class of dyes known as phthalein dyes. The addition of a base sodium carbonate reacts with the phenolphthalein and produces a red diazo reaction that is easily visible. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8. Since phenolphthalein is colourless in acid and intensely red in base it is commonly used as a ph indicator in titrations and also as the active ingredient.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If hydrogen ions h as found in an acid were added to the pink solution the equilibrium would switch and the solution would be colorless. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles. Chelonae which give a positive reaction from other rapid growers. It is a weak acid which can lose h ions. Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator.
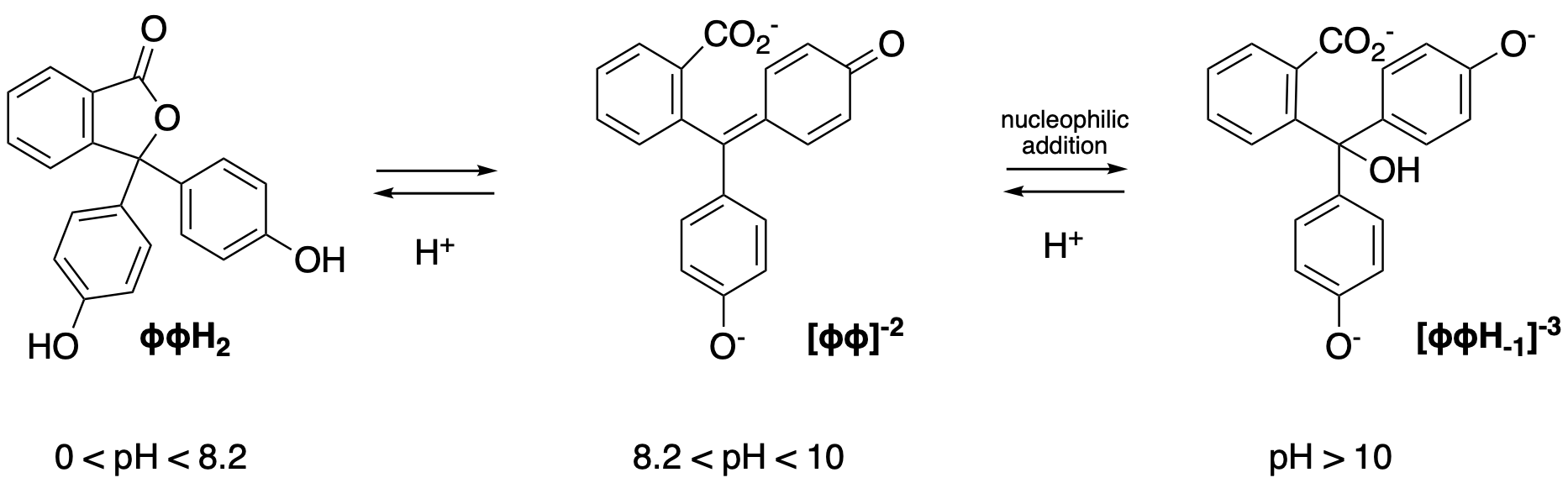 Source: hackuarium.github.io
Source: hackuarium.github.io
As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. But the function of the ph indicator is far advanced. Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. It is an organic compound that we use as an acid or base indicator. If hydrogen ions h as found in an acid were added to the pink solution the equilibrium would switch and the solution would be colorless.
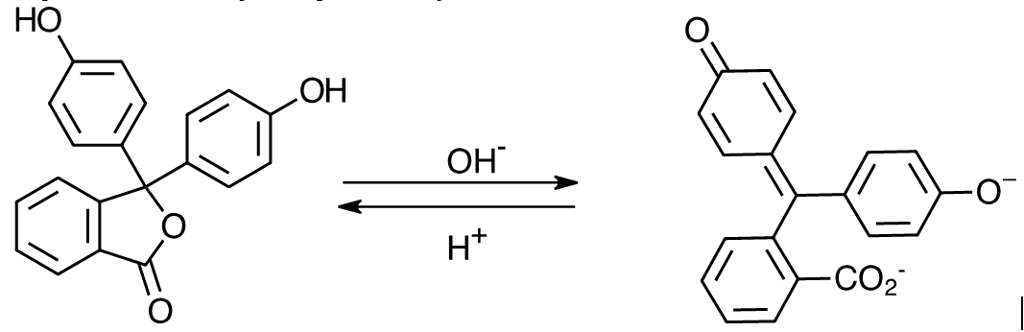 Source: chegg.com
Source: chegg.com
It belongs to the class of dyes known as phthalein dyes. Furthermore the compound is pinkish in basic solution and colourless in acidic solution. For this application it turns colorless in acidic solutions and pink in basic solutions. It is a weak acid which can lose h ions. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles.
 Source: chemicalbook.com
Source: chemicalbook.com
Furthermore the compound is pinkish in basic solution and colourless in acidic solution. Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a ph around 8. Furthermore one of the ph testers is phenolphthalein which is usually colourless but on coming in contact with aid or base changes its colour from pink acidic to purple base. As base is added from the buret to the beaker left the equilibrium shifts from the protonated molecule colorless center to the deprotonated molecule magenta right. Since phenolphthalein is colourless in acid and intensely red in base it is commonly used as a ph indicator in titrations and also as the active ingredient.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
The 3 day test is used to identify and distinguish some rapid growers m. It is an organic compound that we use as an acid or base indicator. Phenolphthalein is an organic compound used as a laboratory reagent and ph indicator. But the function of the ph indicator is far advanced. Furthermore the compound is pinkish in basic solution and colourless in acidic solution.
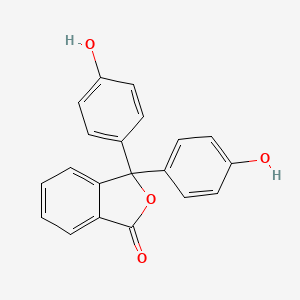
Phenolphthalein is a chemical compound with the formula c20h14o4 and is often written as hin or phph in shorthand notation. Furthermore the compound is pinkish in basic solution and colourless in acidic solution. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles. Phenolphthalein is a weak acid and is colorless in solution although its ion is pink. However phenolphthalein is no longer used as a laxative due to the suspected carcinogenicity of this compound.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phenolphthalein reaction with base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.