Polarity of the water molecule
Polarity Of The Water Molecule. First the polarity of the water molecule and its hydrogen bonding properties. Attracted to one another because the positive area of one molecule is attracted to the negative area of another. Polarity of a water molecule. One of water s important properties is that it is composed of polar molecules.
 Solved Explain How The Unique Properties Of Water Result From Chegg Com From chegg.com
Solved Explain How The Unique Properties Of Water Result From Chegg Com From chegg.com
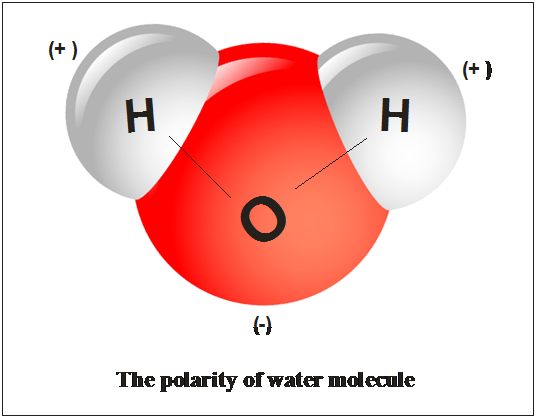
Polarity in water arises due to partial negative δ charge near the oxygen atom due to unshared pair of electrons and partial positive charge δ near the hydrogen atom. Oxygen has a stronger attraction for electrons than hydrogen. When solutes are added to water they may be affected by the charge distribution. Water h 2 o is polar because of the bent shape of the molecule. The molecule has an overall neutral charge because the two charges cancel each other out but the charges are not evenly distributed across the molecule. A water molecule consists of one oxygen atom and two hydrogen atoms.
While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen.
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Polarity of water definition. Attracted to one another because the positive area of one molecule is attracted to the negative area of another. Slightly negative near the oxygen atom slightly positive near the hydrogen atoms. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. First the polarity of the water molecule and its hydrogen bonding properties.
 Source: sciencenotes.org
Source: sciencenotes.org
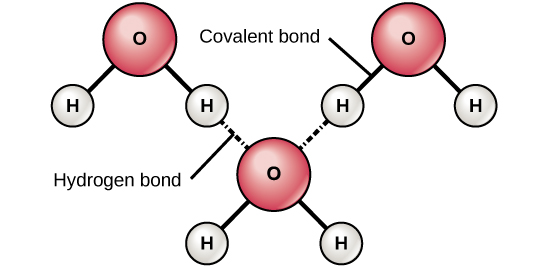
This uneven distribution of electron density on a water molecule makes it a polar molecule. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. The water molecule consists of two hydrogen atoms joined to a single oxygen atom by a covalent bond. One of water s important properties is that it is composed of polar molecules. Attracted to one another because the positive area of one molecule is attracted to the negative area of another.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Water is a multimolecule comprising of various molecules of water bonded to one another. This causes on end of the molecule to be negative while the other is positive. Oxygen has a very high electronegativity meaning it has a very high affinity for electrons. The water molecule consists of two hydrogen atoms joined to a single oxygen atom by a covalent bond. Attracted to one another because the positive area of one molecule is attracted to the negative area of another.
 Source: biology.arizona.edu
Source: biology.arizona.edu
This is an example of polar covalent chemical bonding. Attracted to one another because the positive area of one molecule is attracted to the negative area of another. First the polarity of the water molecule and its hydrogen bonding properties. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. Polarity of a water molecule.
 Source: khanacademy.org
Source: khanacademy.org
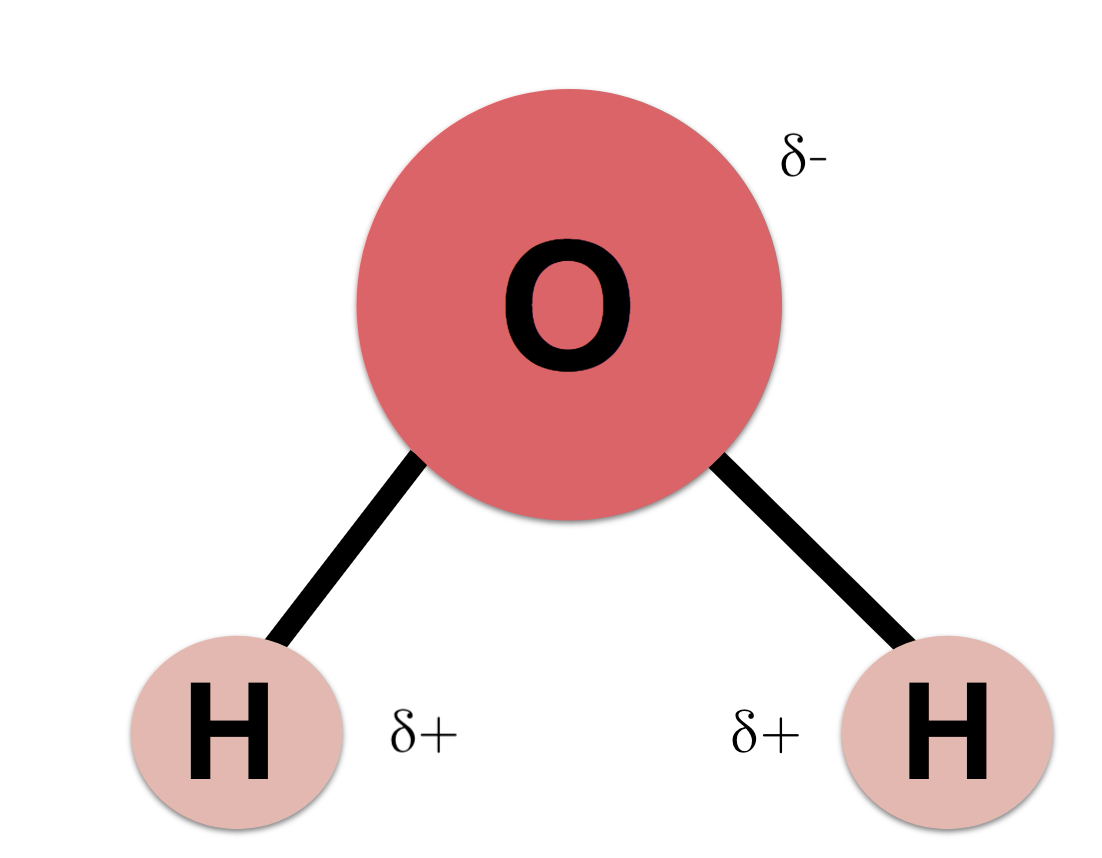
Oxygen has a stronger attraction for electrons than hydrogen. Water h 2 o is polar because of the bent shape of the molecule. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. A water molecule consists of one oxygen atom and two hydrogen atoms. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
 Source: usgs.gov
Source: usgs.gov
Attracted to one another because the positive area of one molecule is attracted to the negative area of another. Polarity in water arises due to partial negative δ charge near the oxygen atom due to unshared pair of electrons and partial positive charge δ near the hydrogen atom. This uneven distribution of electron density on a water molecule makes it a polar molecule. The water molecule consists of two hydrogen atoms joined to a single oxygen atom by a covalent bond. Molecules of water are attracted to each other through these differences in polarity forming the important hydrogen bonds that give water many of its unique properties.
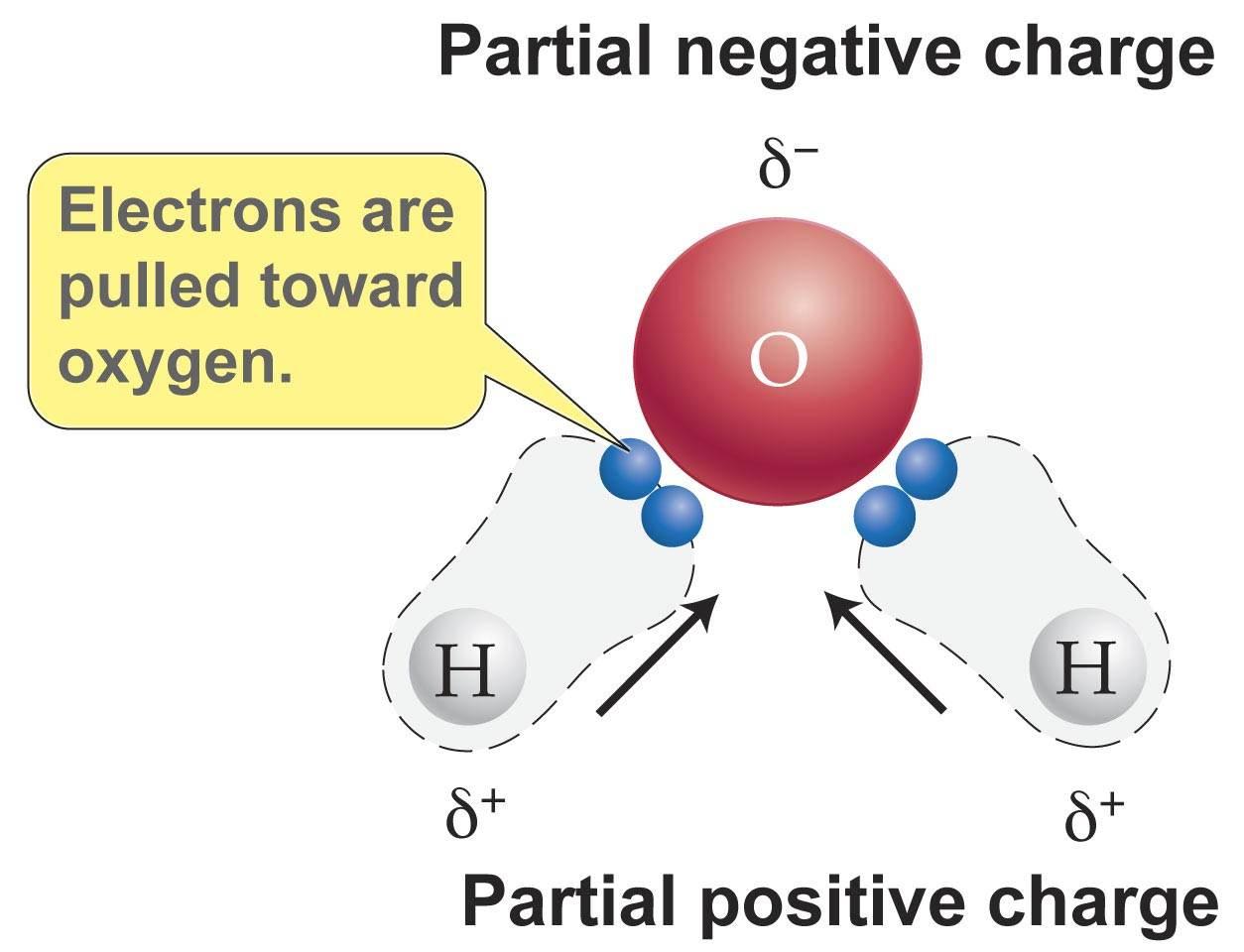 Source: socratic.org
Source: socratic.org
One of water s important properties is that it is composed of polar molecules. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Attracted to one another because the positive area of one molecule is attracted to the negative area of another. The molecule has an overall neutral charge because the two charges cancel each other out but the charges are not evenly distributed across the molecule. This uneven distribution of electron density on a water molecule makes it a polar molecule.
 Source: usgs.gov
Source: usgs.gov
The water molecule consists of two hydrogen atoms joined to a single oxygen atom by a covalent bond. The water molecule h 2 o is a polar molecule with a strong tendency to form hydrogen bonds. One of water s important properties is that it is composed of polar molecules. Polarity of a water molecule. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen.
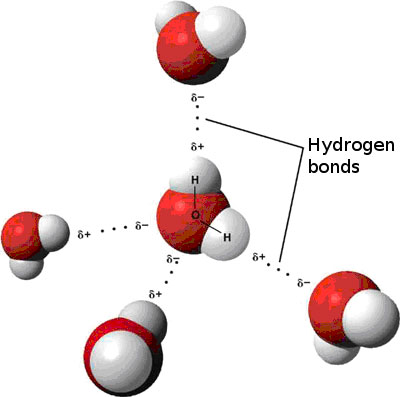 Source: sciencepartners.info
Source: sciencepartners.info
Polarity of water definition. The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative. A water molecule consists of one oxygen atom and two hydrogen atoms. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Polarity of water definition.
 Source: chegg.com
Source: chegg.com
This is an example of polar covalent chemical bonding. Oxygen has a stronger attraction for electrons than hydrogen. When solutes are added to water they may be affected by the charge distribution. Water is a multimolecule comprising of various molecules of water bonded to one another. This is an example of polar covalent chemical bonding.
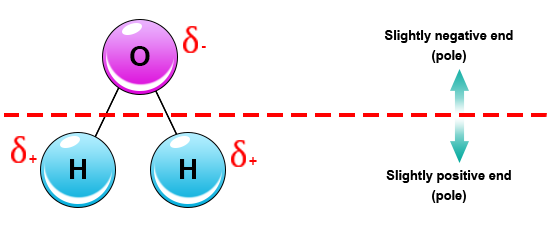 Source: socratic.org
Source: socratic.org
Attracted to one another because the positive area of one molecule is attracted to the negative area of another. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. The water molecule consists of two hydrogen atoms joined to a single oxygen atom by a covalent bond. Polarity of a water molecule. The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative.
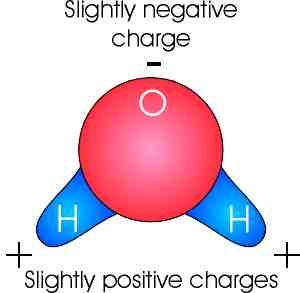 Source: marinebiology.org
Source: marinebiology.org
This causes on end of the molecule to be negative while the other is positive. The water molecule h 2 o is a polar molecule with a strong tendency to form hydrogen bonds. Polarity of a water molecule. First the polarity of the water molecule and its hydrogen bonding properties. Polarity of water definition.
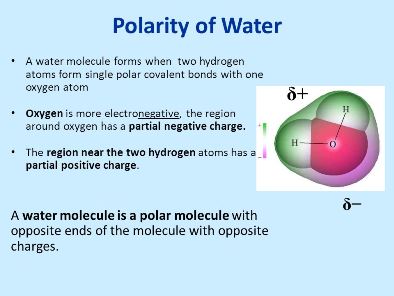 Source: masteringbiologyquiz.com
Source: masteringbiologyquiz.com
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Molecules of water are attracted to each other through these differences in polarity forming the important hydrogen bonds that give water many of its unique properties. Polarity of a water molecule. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
 Source: newwatermodel.blogspot.com
Source: newwatermodel.blogspot.com
First the polarity of the water molecule and its hydrogen bonding properties. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. Oxygen has a very high electronegativity meaning it has a very high affinity for electrons. The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative. Polarity of water definition.
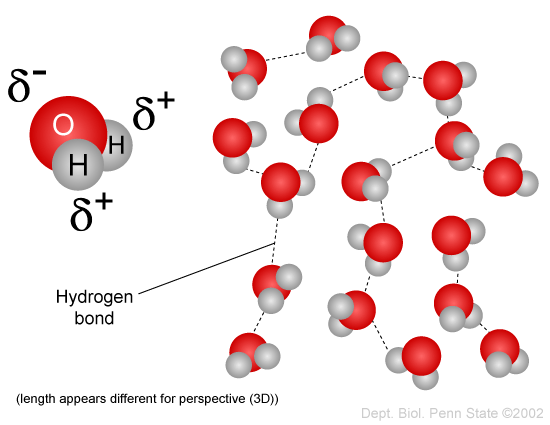 Source: sphweb.bumc.bu.edu
Source: sphweb.bumc.bu.edu
This uneven distribution of electron density on a water molecule makes it a polar molecule. Polarity of a water molecule. Attracted to one another because the positive area of one molecule is attracted to the negative area of another. Molecules of water are attracted to each other through these differences in polarity forming the important hydrogen bonds that give water many of its unique properties. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly.
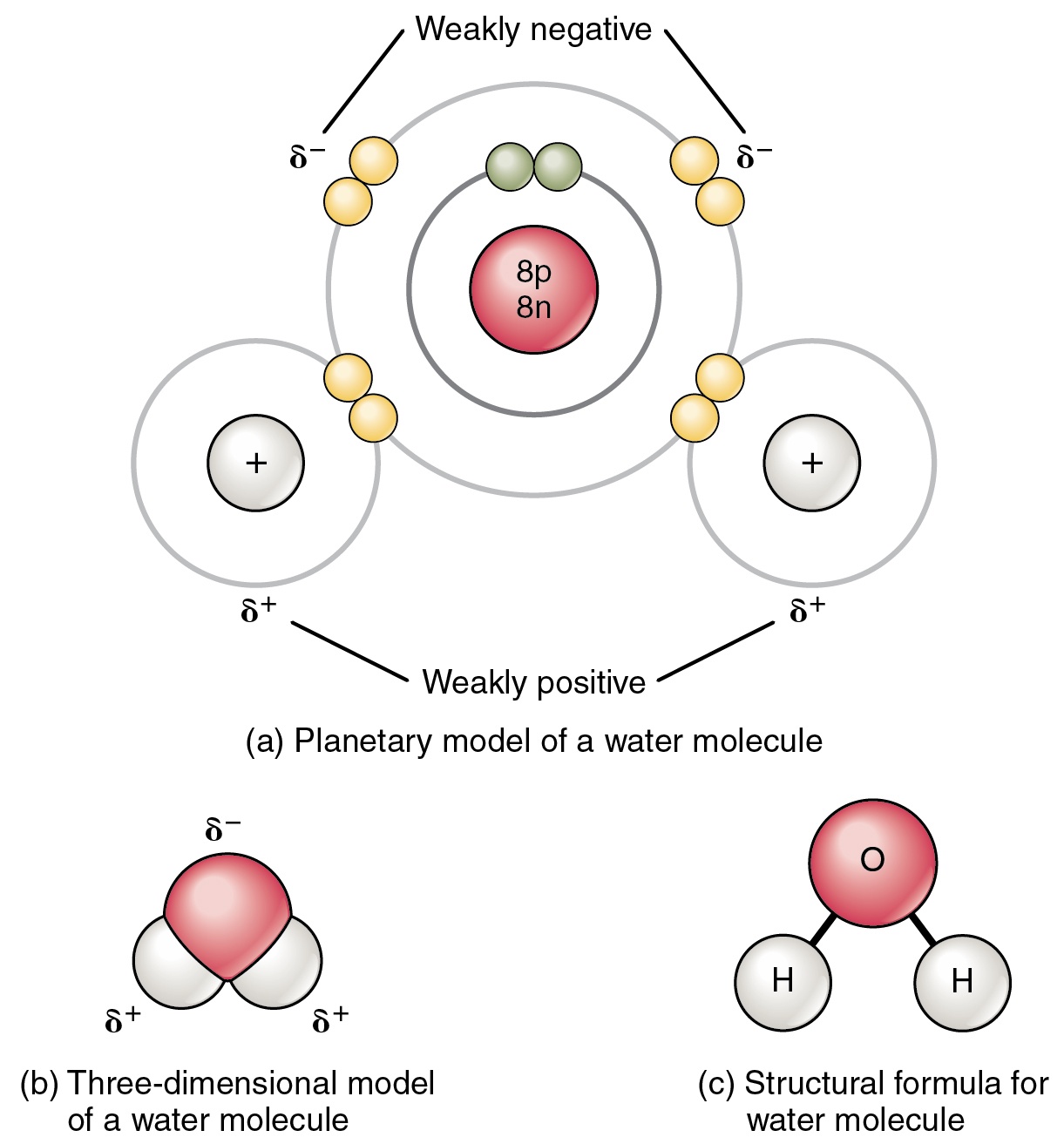 Source: expii.com
Source: expii.com
The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule. The water molecule h 2 o is a polar molecule with a strong tendency to form hydrogen bonds. Water h 2 o is polar because of the bent shape of the molecule. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. This causes on end of the molecule to be negative while the other is positive.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title polarity of the water molecule by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.