Polarity of water definition
Polarity Of Water Definition. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. Lipid soluble nonpolar molecules pass readily through the membrane because they dissolve in the hydrophobic nonpolar portion of the lipid bilayer. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving.

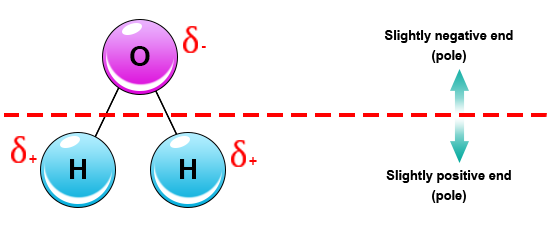
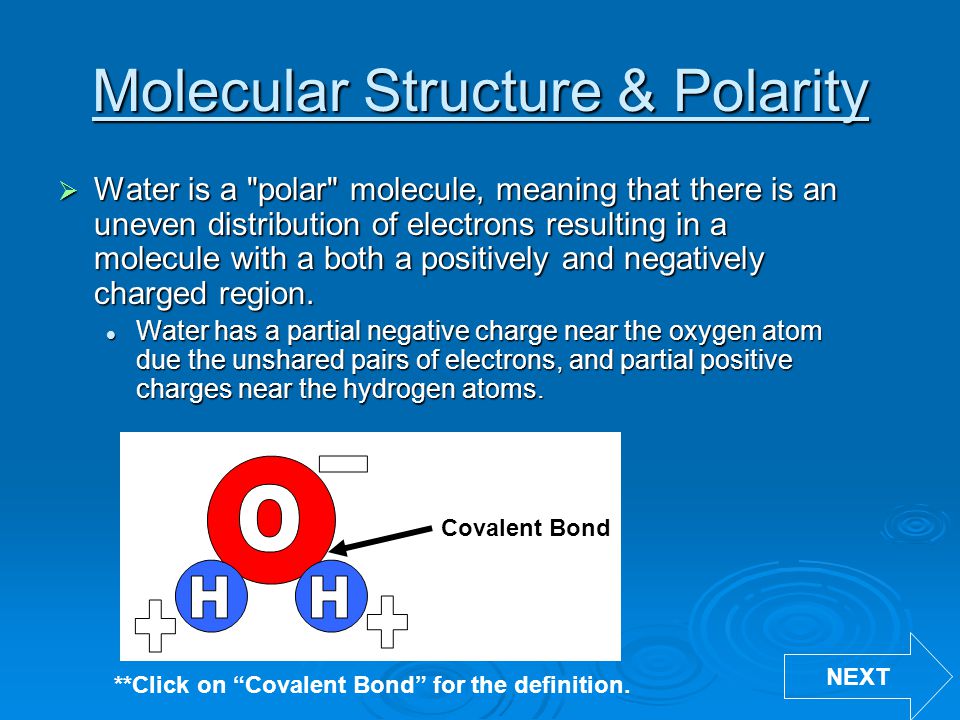
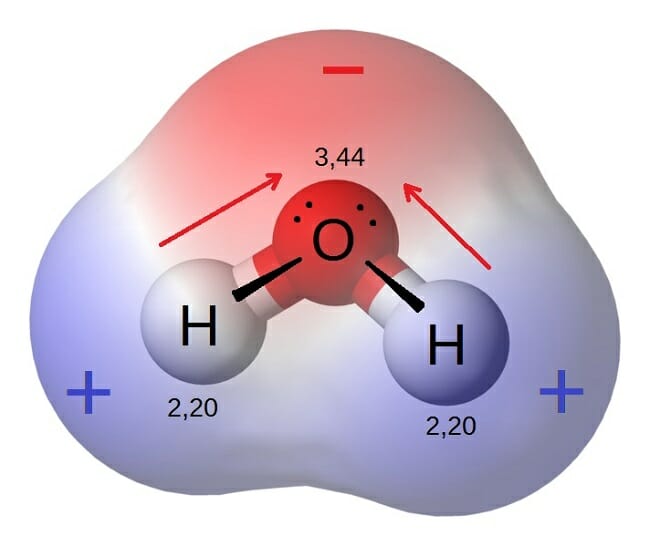
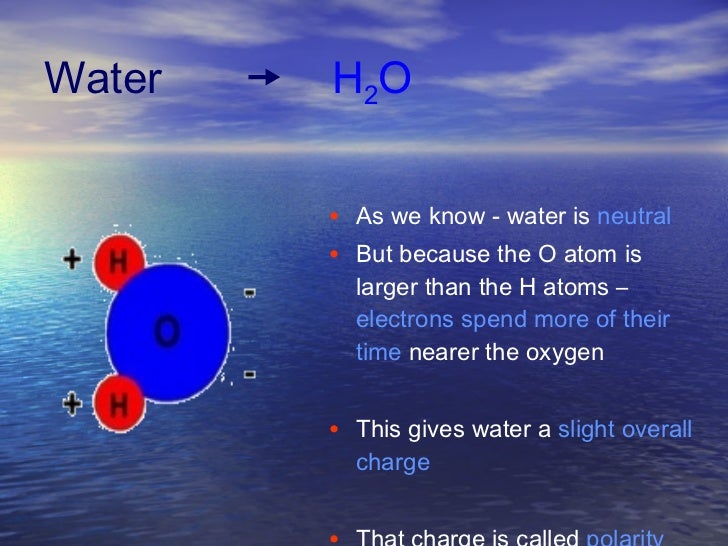
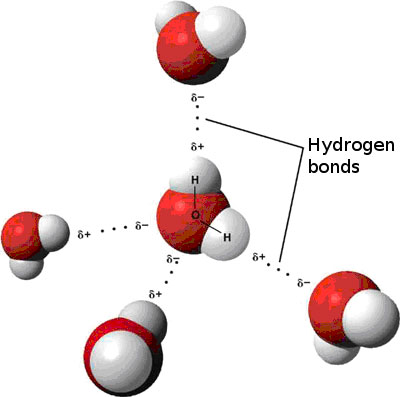
The outcome is a difference in charge or polarity from one end of the molecule to the other. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms. This causes on end of the molecule to be negative while the other is positive. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen.
While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction.
Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. This causes on end of the molecule to be negative while the other is positive. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction.
 Source: wyzant.com
Source: wyzant.com
Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Although permeable to water a polar molecule the nonpolar lipid bilayer of. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving.
 Source: masteringbiologyquiz.com
Source: masteringbiologyquiz.com
This causes on end of the molecule to be negative while the other is positive. This causes on end of the molecule to be negative while the other is positive. Lipid soluble nonpolar molecules pass readily through the membrane because they dissolve in the hydrophobic nonpolar portion of the lipid bilayer. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms.
 Source: biology.arizona.edu
Source: biology.arizona.edu
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. This causes on end of the molecule to be negative while the other is positive. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving.

Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. This causes on end of the molecule to be negative while the other is positive. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms.
 Source: socratic.org
Source: socratic.org
While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen.
 Source: m.youtube.com
Source: m.youtube.com
Although permeable to water a polar molecule the nonpolar lipid bilayer of. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms. The outcome is a difference in charge or polarity from one end of the molecule to the other. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom.
 Source: slideplayer.com
Source: slideplayer.com
Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Although permeable to water a polar molecule the nonpolar lipid bilayer of.
 Source: sciencenotes.org
Source: sciencenotes.org
A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. Although permeable to water a polar molecule the nonpolar lipid bilayer of. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction.
 Source: biologydictionary.net
Source: biologydictionary.net
Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The outcome is a difference in charge or polarity from one end of the molecule to the other. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. This causes on end of the molecule to be negative while the other is positive. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly.
 Source: slideshare.net
Source: slideshare.net
A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Although permeable to water a polar molecule the nonpolar lipid bilayer of. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. This causes on end of the molecule to be negative while the other is positive.
 Source: sciencepartners.info
Source: sciencepartners.info
Although permeable to water a polar molecule the nonpolar lipid bilayer of. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. Although permeable to water a polar molecule the nonpolar lipid bilayer of. The outcome is a difference in charge or polarity from one end of the molecule to the other. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction.
 Source: sciencenotes.org
Source: sciencenotes.org
Although permeable to water a polar molecule the nonpolar lipid bilayer of. This causes on end of the molecule to be negative while the other is positive. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Nonpolar chemicals are considered lipophilic lipid loving and polar chemicals are hydrophilic water loving.
 Source: ifsa.my
Source: ifsa.my
The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms. Lipid soluble nonpolar molecules pass readily through the membrane because they dissolve in the hydrophobic nonpolar portion of the lipid bilayer. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The polarity of water is the net result of the overall negative charge of the oxygen atom and the overall positive charges of the two hydrogen atoms.
 Source: britannica.com
Source: britannica.com
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Water s charges are generated because oxygen is more electronegative or electron loving than hydrogen.
 Source: www2.estrellamountain.edu
Source: www2.estrellamountain.edu
Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The outcome is a difference in charge or polarity from one end of the molecule to the other. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title polarity of water definition by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.