What causes polarity in a water molecule
What Causes Polarity In A Water Molecule. This is an example of polar covalent chemical bonding. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. A water molecule because of its shape is a polar molecule that is it has one side that is positively charged and one side that is negatively charged. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly.
 Why Is Water A Polar Molecule Socratic From socratic.org
Why Is Water A Polar Molecule Socratic From socratic.org
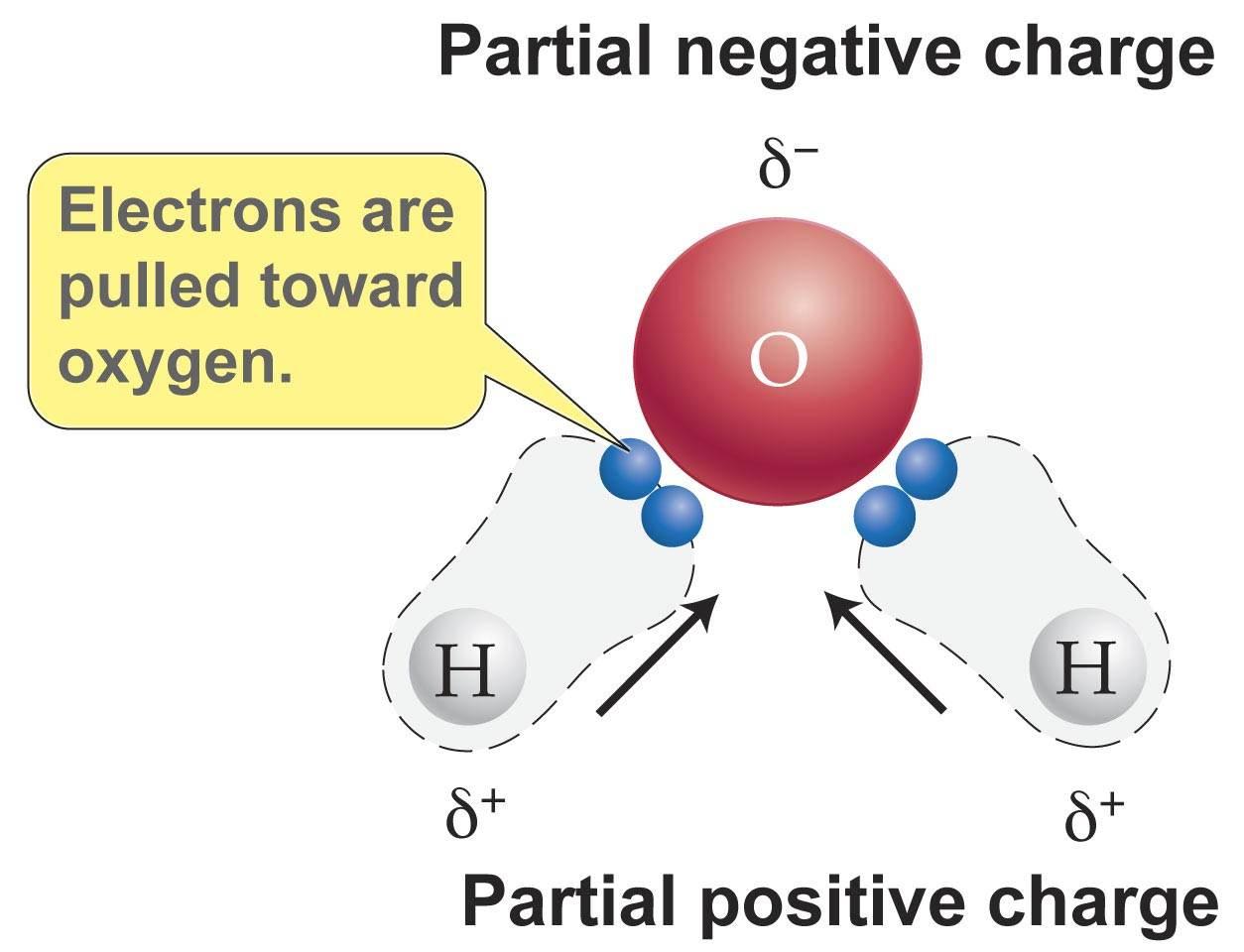
This phenomenon can be explained by the polarity of water. These effects add as vectors to make the overall molecule polar. This creates a distinct region of negative charge around the oxygen atom and a pair of distinctly positive regions around each hydrogen atom on same side of molecule. Water h 2 o is polar because of the bent shape of the molecule. In turn this electronegativity causes the bend in the water molecule and thus its. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20.
When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds.
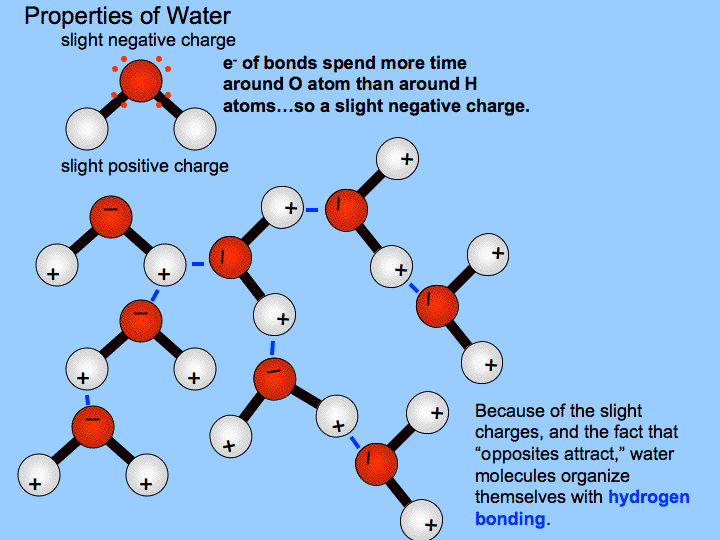
Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. Polarity of a water molecule. This phenomenon can be explained by the polarity of water. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom.
 Source: usgs.gov
Source: usgs.gov
This phenomenon can be explained by the polarity of water. This phenomenon can be explained by the polarity of water. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. This creates a distinct region of negative charge around the oxygen atom and a pair of distinctly positive regions around each hydrogen atom on same side of molecule.
 Source: slideplayer.com
Source: slideplayer.com
This phenomenon can be explained by the polarity of water. This phenomenon can be explained by the polarity of water. The electronegativity difference polarizes each h o bond shifting its electrons towards the oxygen illustrated by red arrows. These effects add as vectors to make the overall molecule polar. Water is polar because of the bent shape of the molecule.
 Source: sciencenotes.org
Source: sciencenotes.org
This is an example of polar covalent chemical bonding. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. The electronegativity difference polarizes each h o bond shifting its electrons towards the oxygen illustrated by red arrows. Water is polar because the electronegativity of oxygen is substantially greater than that of hydrogen. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom.
 Source: sites.google.com
Source: sites.google.com
This is an example of polar covalent chemical bonding. The electronegativity difference polarizes each h o bond shifting its electrons towards the oxygen illustrated by red arrows. The greater the difference in electronegativity and the greater the asymmetry the more polar a molecule will be. Water is polar because it has two unshared pairs of electrons on the central oxygen molecule. These effects add as vectors to make the overall molecule polar.
 Source: biology.arizona.edu
Source: biology.arizona.edu
Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. These effects add as vectors to make the overall molecule polar. This causes the single covalent bonds between the. Water is polar because it has two unshared pairs of electrons on the central oxygen molecule.
 Source: slideplayer.com
Source: slideplayer.com
These effects add as vectors to make the overall molecule polar. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The greater the difference in electronegativity and the greater the asymmetry the more polar a molecule will be. This creates a distinct region of negative charge around the oxygen atom and a pair of distinctly positive regions around each hydrogen atom on same side of molecule. This causes on end of the molecule to be negative while the other is positive.
 Source: ausearthed.blogspot.com
Source: ausearthed.blogspot.com
A water molecule because of its shape is a polar molecule that is it has one side that is positively charged and one side that is negatively charged. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water is polar because the electronegativity of oxygen is substantially greater than that of hydrogen. Water expands when it is frozen but is still composed of the same number of molecules thus decreasing its density and allowing it to float in water. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom.
 Source: quizlet.com
Source: quizlet.com
Water expands when it is frozen but is still composed of the same number of molecules thus decreasing its density and allowing it to float in water. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. This phenomenon can be explained by the polarity of water. This causes on end of the molecule to be negative while the other is positive. This is an example of polar covalent chemical bonding.
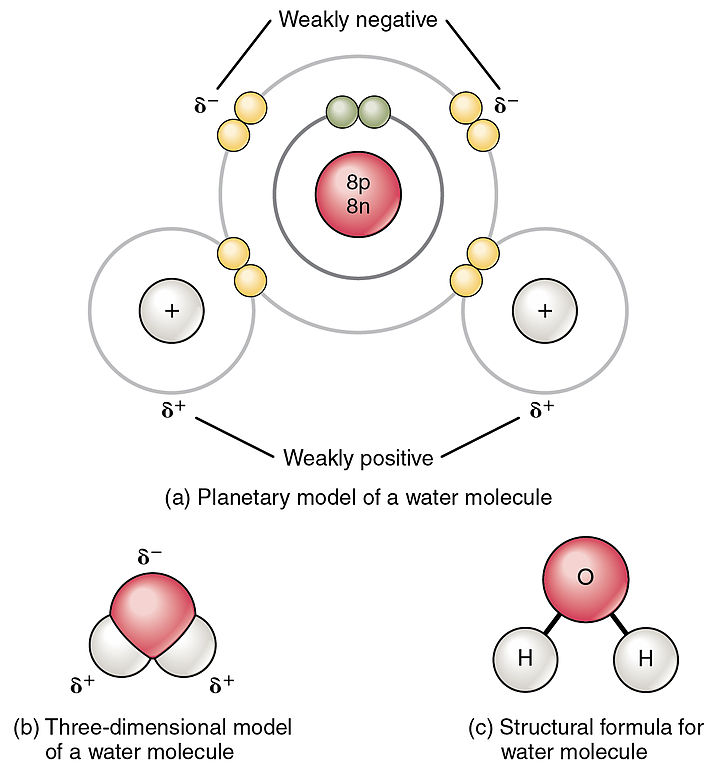 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
Water is polar because it has two unshared pairs of electrons on the central oxygen molecule. The greater the difference in electronegativity and the greater the asymmetry the more polar a molecule will be. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Polarity of a water molecule. This causes the single covalent bonds between the.
 Source: socratic.org
Source: socratic.org
Water h 2 o is polar because of the bent shape of the molecule. A water molecule because of its shape is a polar molecule that is it has one side that is positively charged and one side that is negatively charged. In turn this electronegativity causes the bend in the water molecule and thus its. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20.
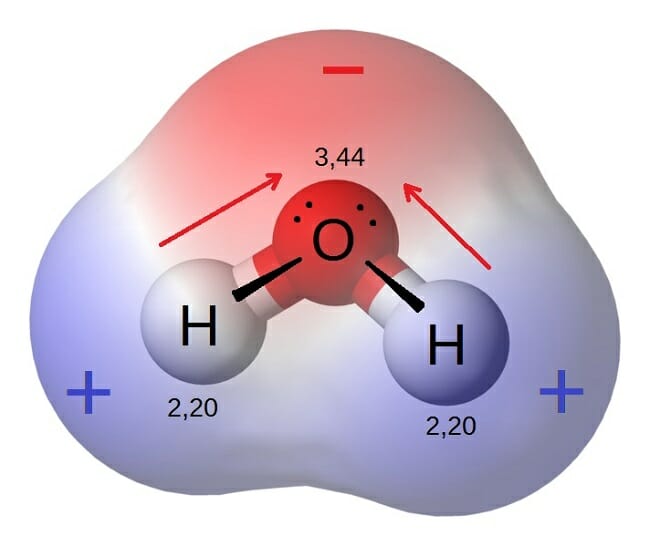 Source: biologydictionary.net
Source: biologydictionary.net
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds. Water is polar because the electronegativity of oxygen is substantially greater than that of hydrogen. This creates a distinct region of negative charge around the oxygen atom and a pair of distinctly positive regions around each hydrogen atom on same side of molecule.
 Source: slideplayer.com
Source: slideplayer.com
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. This causes on end of the molecule to be negative while the other is positive. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. This causes the single covalent bonds between the. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20.
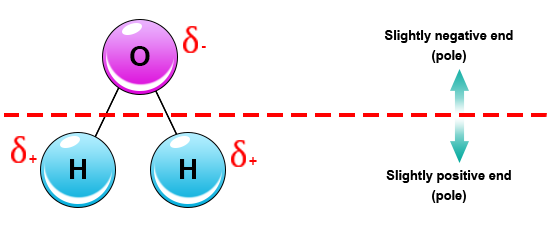 Source: socratic.org
Source: socratic.org
Water is polar because the electronegativity of oxygen is substantially greater than that of hydrogen. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. In turn this electronegativity causes the bend in the water molecule and thus its. A water molecule because of its shape is a polar molecule that is it has one side that is positively charged and one side that is negatively charged. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds.
 Source: sciencenotes.org
Source: sciencenotes.org
What causes a water molecule to have polarity include oxygen s electronegativity in your answer. The electronegativity difference polarizes each h o bond shifting its electrons towards the oxygen illustrated by red arrows. What causes a water molecule to have polarity include oxygen s electronegativity in your answer. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20. This causes on end of the molecule to be negative while the other is positive.
 Source: slideplayer.com
Source: slideplayer.com
This causes on end of the molecule to be negative while the other is positive. This phenomenon can be explained by the polarity of water. Polarity of the molecule is driven by electronegativity with the oxygen in water as a prime example. The water molecule is made up of oxygen and hydrogen with respective electronegativities of 3 44 and 2 20. This creates a distinct region of negative charge around the oxygen atom and a pair of distinctly positive regions around each hydrogen atom on same side of molecule.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what causes polarity in a water molecule by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.