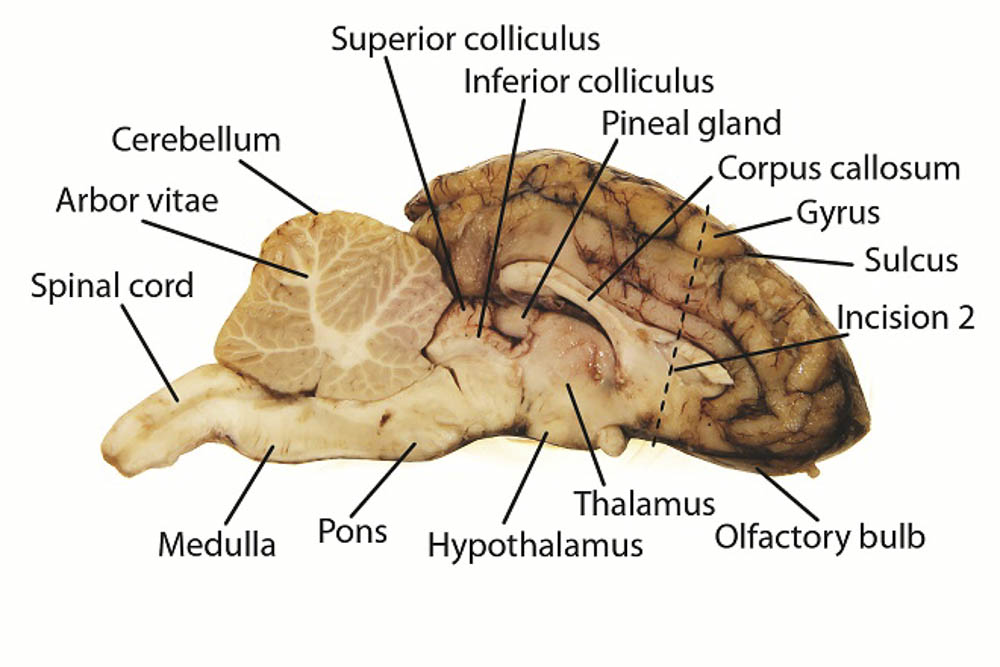
What causes polarity in water molecules
What Causes Polarity In Water Molecules. Polarity of a water molecule water h2o is polar because of the bent shape of the molecule. This is an example of polar covalent chemical bonding. An uneven charge between the oxygen molecule and 2 hydrogen molecules. Polarity is mainly caused by electronegative between atoms in a molecule.
 The Polarity Of Water Mastering Biology Quiz From masteringbiologyquiz.com
The Polarity Of Water Mastering Biology Quiz From masteringbiologyquiz.com
Polarity of a water molecule water h2o is polar because of the bent shape of the molecule. Water molecules are a good example of polarity where there is unequal sharing of electrons. Water is a very common substance on earth but not on other planets. The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen. An uneven charge between the oxygen molecule and 2 hydrogen molecules. The highly electronegative oxygen atom attracts electrons or negative charge to it making the region around the oxygen more negative than the areas around the two hydrogen atoms.
An uneven charge between the oxygen molecule and 2 hydrogen molecules.
This is an example of polar covalent chemical bonding. This is an example of polar covalent chemical bonding. Water is a very common substance on earth but not on other planets. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The highly electronegative oxygen atom attracts electrons or negative charge to it making the region around the oxygen more negative than the areas around the two hydrogen atoms. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
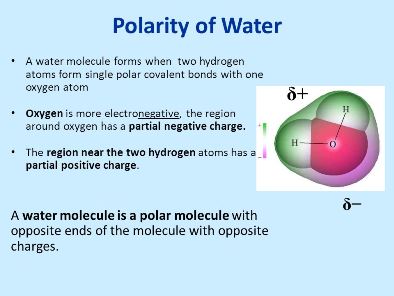
 Source: ces.fau.edu
Source: ces.fau.edu
Polarity of a water molecule water h2o is polar because of the bent shape of the molecule. The polarity of the o h bonds and the asymmetry of the molecule. Water s polarity is what gives water its unique properties. Simply so what causes polarity in a water molecule. Polarity of a water molecule water h2o is polar because of the bent shape of the molecule.
 Source: quizlet.com
Source: quizlet.com
Water has the chemical formula h 2 o. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds. The polarity of the o h bonds and the asymmetry of the molecule. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Water s polarity is what gives water its unique properties.
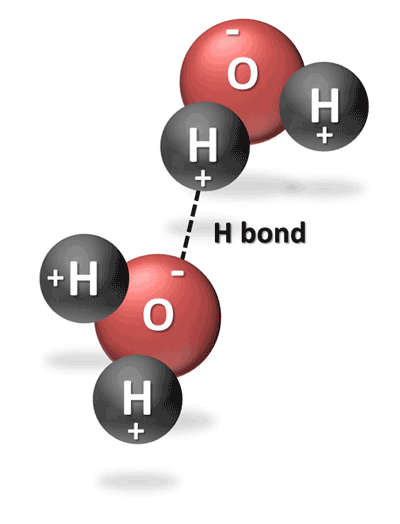
 Source: expii.com
Source: expii.com
This causes on end of the molecule to be negative while the other is positive. The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen. The polarity of the o h bonds and the asymmetry of the molecule. An uneven charge between the oxygen molecule and 2 hydrogen molecules. Water molecules are a good example of polarity where there is unequal sharing of electrons.
 Source: usgs.gov
Source: usgs.gov
Polarity of a water molecule water h 2 o is polar because of the bent shape of the molecule. An uneven charge between the oxygen molecule and 2 hydrogen molecules. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Water is a very common substance on earth but not on other planets.
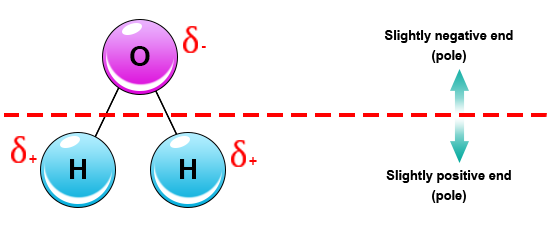 Source: socratic.org
Source: socratic.org
The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The highly electronegative oxygen atom attracts electrons or negative charge to it making the region around the oxygen more negative than the areas around the two hydrogen atoms. Polarity is mainly caused by electronegative between atoms in a molecule. The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen.
 Source: biology.arizona.edu
Source: biology.arizona.edu
The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. An uneven charge between the oxygen molecule and 2 hydrogen molecules. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom.
 Source: masteringbiologyquiz.com
Source: masteringbiologyquiz.com
Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. This phenomenon can be explained by the polarity of water. The polarity of the o h bonds and the asymmetry of the molecule. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. Water is a very common substance on earth but not on other planets.
 Source: sciencenotes.org
Source: sciencenotes.org
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water molecules are a good example of polarity where there is unequal sharing of electrons. Water is a very common substance on earth but not on other planets. The lone pairs on the oxygen molecule create a bent shape the valence electron pairs repel the o h bonds folding them together at an angle of 104 5. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons.
 Source: sciencenotes.org
Source: sciencenotes.org
Simply so what causes polarity in a water molecule. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds. The lone pairs on the oxygen molecule create a bent shape the valence electron pairs repel the o h bonds folding them together at an angle of 104 5. The polarity of the o h bonds and the asymmetry of the molecule. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly.
 Source: slideplayer.com
Source: slideplayer.com
The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen. Water is a very common substance on earth but not on other planets. The lone pairs on the oxygen molecule create a bent shape the valence electron pairs repel the o h bonds folding them together at an angle of 104 5. Water s polarity is what gives water its unique properties. This is an example of polar covalent chemical bonding.
 Source: slideplayer.com
Source: slideplayer.com
The polarity of the o h bonds and the asymmetry of the molecule. Polarity of a water molecule water h2o is polar because of the bent shape of the molecule. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. This is an example of polar covalent chemical bonding. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds.
 Source: slideplayer.com
Source: slideplayer.com
An uneven charge between the oxygen molecule and 2 hydrogen molecules. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. An uneven charge between the oxygen molecule and 2 hydrogen molecules. Water molecules are a good example of polarity where there is unequal sharing of electrons. This causes on end of the molecule to be negative while the other is positive.
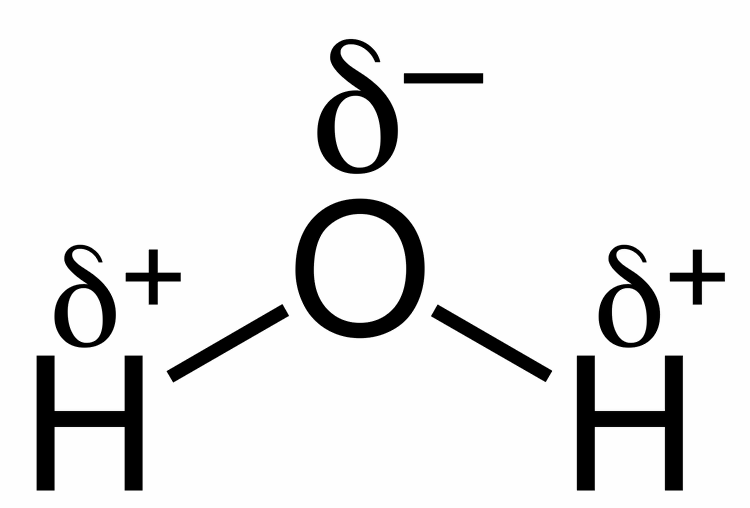 Source: alevelbiology.co.uk
Source: alevelbiology.co.uk
Water s polarity is what gives water its unique properties. The polarity of the o h bonds and the asymmetry of the molecule. Water is a very common substance on earth but not on other planets. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The highly electronegative oxygen atom attracts electrons or negative charge to it making the region around the oxygen more negative than the areas around the two hydrogen atoms.
 Source: sites.google.com
Source: sites.google.com
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Polarity is mainly caused by electronegative between atoms in a molecule. When ice is frozen the water molecules extend themselves as far as they possibly can but are held firmly together by hydrogen bonds. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
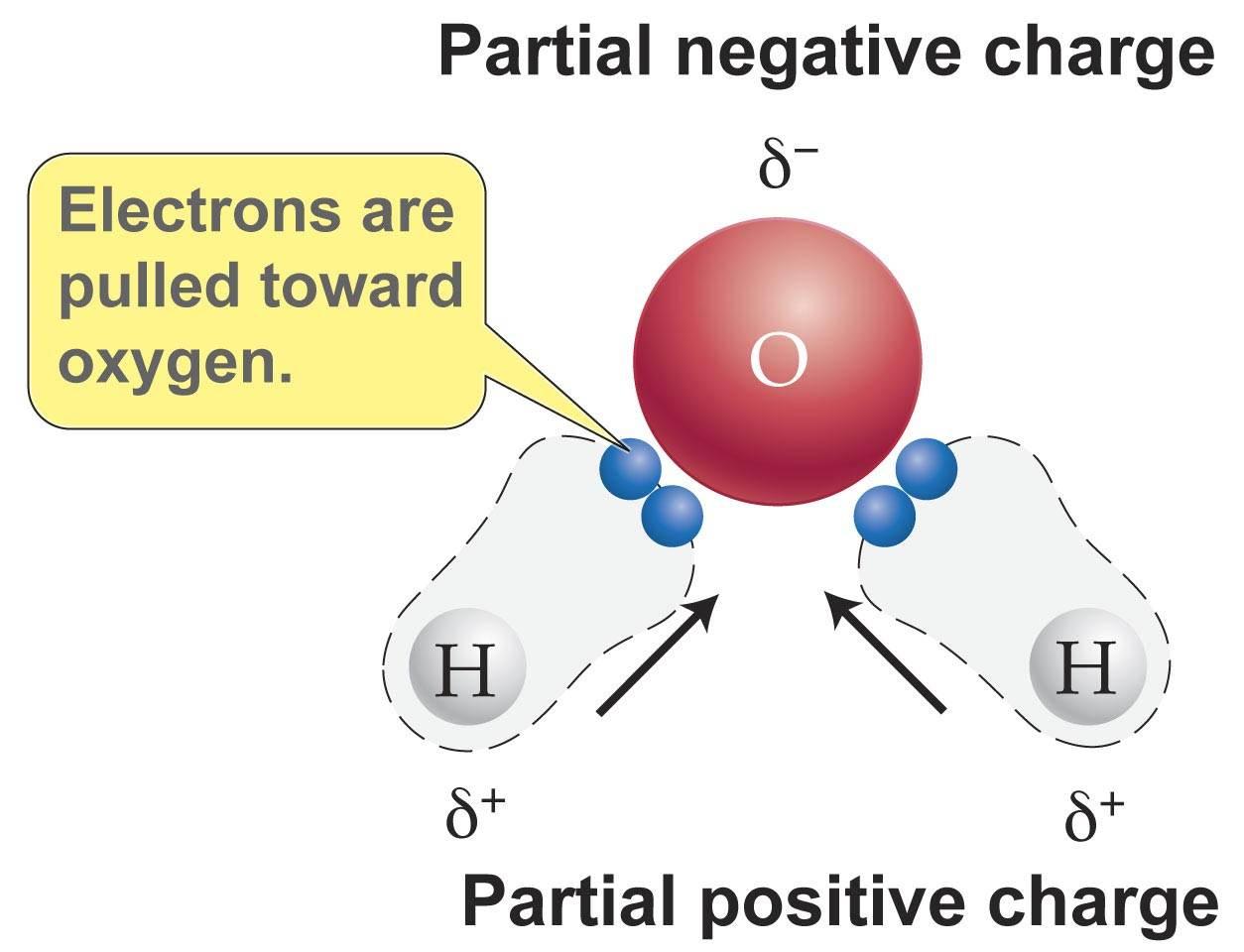 Source: socratic.org
Source: socratic.org
Water s polarity is what gives water its unique properties. An uneven charge between the oxygen molecule and 2 hydrogen molecules. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Polarity is mainly caused by electronegative between atoms in a molecule. The o h bond has a dipole moment of 1 4 a strongly polar bond creating partial charges neg on oxygen positive on hydrogen.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what causes polarity in water molecules by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.